Anticipated Severe Corrosion Problems Associated with the Change from Single Hulled Bulk Carriers to Double Hulled Bulk Carrier Designs
Amtec Consultants Ltd
December 2003
1. Background
The grounding and subsequent hull rupture of the single hulled tanker “Exxon Valdez” resulted in massive pollution of the Alaskan coastline by crude oil. Congress in the USA legislated the use of double hulled tankers to help contain the cargo in the event of grounding or other hull rupture. Recent tanker disasters with older single hulled tankers have re-enforced this anti-pollution viewpoint.
A spin-off effect has led to the proposed introduction of double hulled bulk carrier designs. There is however, a very considerable difference between the operating conditions found on double hulled tankers and bulk carriers.
In general, bulk carriers transport cargo that is non-polluting and many have a tendency to sink rapidly and without trace. When these unpredicted hull breaches occur with single hulled bulk carriers, they can often be the result of the extremely high corrosion rates associated with certain cargoes. Cycling between iron ore and coal has been found to be a contributing factor to these unexpected sinkings.
This document seeks to explore how these existing corrosion problems may very well be made significantly worse by the prospective change from single hulled to double hulled bulk carrier designs.
2. Thermos Effect
After the current designs of double hulled tankers were introduced, it was found that when hot cargoes were loaded at temperatures in the order of 45oC, there was only a small drop in the temperature of the cargo after a long voyage to North latitude ports.
Previous experience with single hulled tanker designs had showed that the cargo quickly cooled and the cargo temperature was approaching that of the sea temperature after half of the voyage. The reason for this large difference in temperature behaviour was that for the double hulled designs, the empty ballast space completely surrounded the cargo with an insulating jacket of air, preventing direct heat transfer through the hull metal between the cargo and the sea. This is the principle used for the thermos flask or vacuum bottle, hence the “thermos effect”.
The humid, high temperature air in the double hulled vessels ballast tanks has also resulted in more rapid corrosion and coating failures in these areas. This is because, in adverse circumstances, the rate of the corrosion reaction doubles with every 10oC rise in temperature. In the worst cases, this could cause a four times increase in the rate of corrosion. However, the corrosion rate is normally under the control of a diffusion reaction, once some build up of rust has occurred.
The coated area of the ballast tanks is very much greater in the double hulled design vessels and economic refurbishment of the early double hulled tankers at ages of greater than ten years is proving to be a great problem.
The corrosion within the cargo tanks of double hulled tankers has been similarly affected. Previously, with single hulled designs, the tank top and under deck areas could be left uncoated, but with the double hull designs, the tank top shows pitting and grooving corrosion much more rapidly (photograph 1) and severe under deck corrosion also occurs (photograph 2), necessitating coating.
The “Thermos” effect will undoubtedly occur with double hulled bulk carriers. Where damp and humid cargoes, such as grain and ore are loaded, corrosion rates will be significantly higher than normal. In the case of damp, “active” cargoes such as coal or petcoke, the anticipated corrosion rates will be extremely high at sites of coating damage or removal.
In the case of double hulled tankers, the thermos effect tends to be evenly spread across the entire cargo tank, for double hulled bulk carriers this will not be the case, due to cargo compaction effects and space that remains empty above the cargo. This will result in thermal gradients within the bulkheads of the cargo holds. Any “hot spots” will become strong anodic sites and suffer from both enhanced corrosion and coating breakdown. Both processes will occur in the cargo holds and in the ballast tanks at the same location.
3. Temperature effect on Corrosion reactions
3.1 Corrosion Processes
Corrosion reactions take place in two stages: a charge transfer step and then a diffusion step. The metal ions have to diffuse away from the reaction site and oxygen or other cathodic reactants have to diffuse to the metal surface.
The initial corrosion reaction is the dissolution of iron from the surface:
Fe -> Fe2+ + 2e-
Within the rust layer, a second reaction occurs that oxidises the ferrous ion to the ferric ion:
3 Fe2+ + 6 OH- -> 3 Fe(OH)2
The electrons produced by the anodic reactions and their intermediate steps are consumed by a cathodic reaction. In neutral solutions, in the absence of other ions or pollutants, this is usually the reduction of oxygen to hydroxyl ions:
O2 + 2 H2O + 4 e- -> 4 OH-
In acidic or polluted conditions, the cathodic reaction is the evolution of hydrogen via the reaction:
2 H+ + 2 e- -> H2
3.2 Corrosion Rates vs Temperature
If the rate of the reaction is determined only by its initial oxidation step, then the corrosion rate increases exponentially with temperature in a manner governed by the Arrhenius expression:
r = A exp (-E / RT)
Where:
r = corrosion rate
A = a constant
E = activation energy
R = gas constant
T = absolute temperature.
Diagram 1 shows a typical example of this type of behaviour for low alloy steel
immersed in dilute hydrochloric acid.

Diagram 1. The effect of temperature on corrosion rate under acid conditions.
The temperature of the steel and the temperature of the environment cannot be
viewed as independent. The specific conductance of seawater increases with temperature.
At 0oC seawater conductance is in the order of 0.023 reciprocal ohm cm, whilst
at 25oC it is 0.042 and continues to change at a similar rate over the region
of interest, 20oC to 50oC. The more conductive that the environment becomes,
the faster the corrosion rate.
3.3 Diffusion Rates vs Temperature
Dissolved oxygen in the seawater also varies with temperature. At 5oC it is 0.65 mg/litre and at 30oC it is in the order of 0.41 mg/litre. The diffusion of oxygen and iron ions through seawater is faster with increasing temperature and is governed by the Nernst equation:
D = RT/F2 { [(V1 + V2) (L1 x L2)] / [V1 Z1 (L1 + L2)] }
Where:
D = diffusion co-efficient.
F = Faraday’s constant.
T = absolute temperature.
L1 and L2 = cation and anion limiting equivalent conductances.
V1 and V2 = number of cations and anions from one molecule of electrolyte (one for NaCl).
Z1 = cation valency (one for NaCl).
From the above equation it can be seen that for diffusion controlled reactions, the corrosion rate would be expected to increase in a linear manner with temperature.
On balance therefore, the effects of temperature on the charge transfer step, the conductivity and the diffusion constant, far outweigh the effects of changes in oxygen solubility.
Water line corrosion is a particularly strong effect of the diffusion of oxygen (photograph 3). Water lines are areas of oxygen super saturation and hence the corrosion rate here is not under diffusion control but under activation (Arrhenius) control. It would be anticipated that static water lines in ballast tanks in double hulled vessels would be prone to the severe effects of this type of corrosion in a similar manner to aft peak tanks located in close proximity to hot engine rooms.
3.4 Surface Area Effects
The reactions are extremely dependent on surface area. The surface area of the cathode is of particular importance. When the cathode is of high surface area, such as a cargo of petcoke, then the rate of the anodic corrosion reaction can be extremely high.
This area effect is not only limited to cargo holds but has caused high corrosion rates in repaired vessels such as recent, near disastrous, structurally damaged vessels due to “accelerating corrosion”, which had high corrosion rates on the repaired area because the older coated areas acted as a large cathode.
Repairs carried out in the void spaces of double hulled bulk carriers are likely to suffer from high corrosion rates for similar reasons and exacerbated by the temperature effect.
3.5 Localised Corrosion
The above discussion has dealt with general, uniform corrosion phenomena. Localised corrosion types are also extremely susceptible to the temperature effect. Pitting corrosion and corrosion fatigue are highly affected by the increased diffusion of oxygen as temperature increases.
3.6 Microbially Induced Corrosion
Localised microbial corrosion reaches a peak around 37oC when the microbes are most active. Anaerobic bacteria, such as sulphate reducing bacteria, follow the Arrhenius equation because the cathodic step:
SO42- + 8 H -> S- + 4 H2O
And the anodic step:
Fe2+ + S2- -> FeS
Are under activation control
3.6 Sacrificial Anodes vs Temperature
The relative corrosion rates of iron and zinc very with temperature in the manner shown in diagram 2.
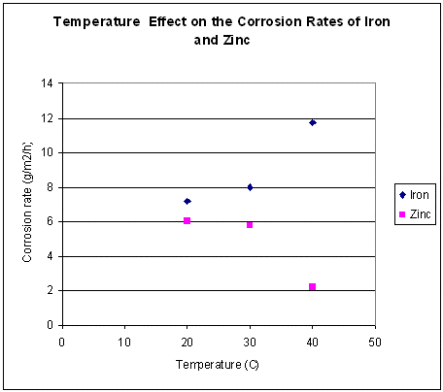
Diagram 2. Corrosion Rates of Iron and Zinc vs Temperature.
The striking feature of this graph is that as the corrosion rate of iron rapidly
increases with increasing temperature, it is mirrored by a corresponding decrease
in the corrosion rate of zinc.
Zinc sacrificial anodes are commonly used to protect ballast tanks and heavy weather ballast holds against corrosion, by corroding preferentially. It can be seen that the implication of the curves of diagram 2 is that over four times the amount of zinc anode surface will be required at 40oC, compared with required at 20oC.
4. Active Cargoes
What is an “active” cargo? For this document, it can be defined as one that physically affects the cargo hold coating and directly influences the corrosion reaction on the underlying steel. Examples of active cargoes in this article are coal, coke, bauxite, sulphur and petcoke. They commonly produce the type of coating breakdown on the corrugated bulkheads of cargo holds that is shown in photograph 4. Rapid corrosion occurs at these damage sites, as shown in photograph 5.
What makes these cargoes “active” is that they are ionically conducting, possess a large surface area with a significantly high moisture content or associated water and the cargoes act as good cathodes.
The corrosion reaction that is taking place on the steel is the anodic half of the process and generates electrons. The electrons are consumed by the reduction reaction of oxygen and water that takes place at the cathodic site. Normally the cathodic reaction is very slow and controls the rate of the anodic dissolution of iron, however, the extremely large surface area of the cargo removes this limitation. The corrosion reaction can occur at significantly increased rates when an active cargo is carried. The corrosion products formed can lever the paint off the steel, causing further fresh steel to be exposed to the aggressive environment. In severe cases, the corrosive solutions that develop can also result in the formation of pits.
Pitting corrosion occurs when there is a small anodic site (in this case the exposed steel) and a large cathodic site. Cathodic sites generally form on the intact coating, but some cargoes are capable of supporting the cathodic reaction. Pitting corrosion can be accelerated with active cargoes, which drives the corrosion process at a greater rate. This is frequently the situation that occurs with carbon based cargoes such as coal and coke. Products such as coke and petcoke have very large surface areas and can form excellent cathodic sites and pits may grow rapidly under such circumstances.
Active cargo corrosion occurs in a sequence of events that results in coating damage, often with the characteristic tree pattern, together with steel corrosion. The first stage of the damage to the paint occurs when the sharp, hard and angular cargo scratches into the coating due to settlement during both loading and the voyage. Eventually the cargo cuts through sites of weak paint to the steel and exposes the metal to the cargo environment.
It is first seen as a small puncture in the coating that creates a pathway for water to reach the steel. Water associated with the cargo can permeate along the interface between the paint and the steel at the site where the coating has been damaged, resulting in the loss of adhesion of the coating.
When examined visually, the damaged area may appear to be small, but when it is investigated with a penknife, it is often found that the paint is loose around the damage site. When this loose coating is removed, the true extent of the corrosion and associated coating delamination becomes apparent. Photograph 6 shows two damage sites that were initially very similar with only tiny punctures visible in the paint. It can be seen that the corrosion under the coating and the loose paint extend several centimetres from the visible damage site.
As the cargo settles, soft or weak paint can move with the cargo creating blisters and “sags” of the type shown in photographs 7 and 8. The loose coating sag is removed by further settlement of the cargo, which in turn can cause the formation of another sag. Repeated “stick – slip” cycles of cargo settlement result in the tree shapes of photograph 9.
5. The Domino Effect and Coating Lifetime Issues in Double Hulled Vessels
Coatings tend to have a reduced service lifetime expectancy at more elevated temperatures. One of the main reasons for this is that they become less elastic more quickly in these circumstances, due to a more rapid loss of residual solvents and an enhanced level of final cure.
In the period immediately following application, epoxy coatings show elongations in the order of 2%, due to the incomplete cure and retained solvents. Over a period of about four years, this becomes reduced to some 0.5% as the level of cure increases and the retained solvents are lost. Coating shrinkage can accompany this effect and results in the coating becoming more easily detached due to internal stress.
At the temperatures in excess of 30oC that are to be anticipated to be more common in double hulled designs, the time to embrittlement of the coating will be approximately halved.
These factors will tend to exacerbate the “Domino Effect” described by Peter Contraros of ABS. In the Domino Effect, coatings breakdown more quickly at highly stressed areas as these areas preferentially become the anodic sites of the corrosion reaction. The rapid corrosion both levers the coating from the metal surface and locally thins the structure. This in turn increases the stress levels.
Localisation of the anodic sites, increased corrosion rates and coating breakdown are all significantly worse at elevated temperatures. It is therefore to be anticipated that the Domino Effect will be a more serious problem in double hulled bulk carriers than in their single hulled equivalents. The relative novelty of the double hull bulk carrier design will make prediction and possibly inspection of this effect problematical.
6. Enhanced Corrosion in Cargo Holds
The combination of the “thermos effect” with “active” cargoes in double hulled bulk carriers is anticipated to lead to extremely high corrosion rates. When this occurs, it will place severe limitations on the operations of such vessels and will limit the vessel’s ability to trade all cargoes world wide.
The carriage of products such as sulphur and sulphurous materials, for example, could become almost impossible, unless the cargo is completely dry. Some active cargoes undergo an exothermic reaction with water, causing their temperature to rise during the course of a voyage. With the single hulled design, the danger of fires in the holds was reduced, as the vertical stiffeners protruding into the cargo tended to act as “heat sinks” and drain the heat away into the surrounding sea water. In the double hulled designs, this will not occur and the danger of very severe corrosion as a result of the significantly higher temperatures, or in the worst case cargo fires, is therefore increased.
Some idea as to the level of accelerated corrosion attack to be expected in the double hulled design, can be obtained from comparing different areas of existing holds that exhibit this problem. When warm cargoes are carried, the corrugated bulkheads between holds experience the same temperature on both sides, often about 40oC. The shell plating has the sea at a much lower temperature on the outside, typically 5-10oC. The face plates of the vertical stiffeners on the shell plating are at an intermediate temperature because they project into the cargo but have a heat sink to the shell plating. In a double hull design, the shell plating and stiffeners would remain at a similarly high temperature as the corrugated bulkheads, allowing an increased rate of corrosion.
Photographs 10 and 11 were taken in the same hold of a bulk carrier that had only carried one cargo. Photograph 10 shows a section of corrugated bulkhead between two holds, with over 200 defects per square metre that are associated with rapid corrosion. Photograph 11 is of a typical area of shell plating at the same height. It can be seen that there is an order of magnitude fewer corrosion sites and that the degree of corrosion is also very much less.
The stiffeners projecting into the cargo are condition that is intermediate between that of the shell plating and the corrugated bulkhead. In the double hulled design, the side plating would be expected to behave in a similar manner to the corrugated bulkheads.
7. Enhanced Corrosion in Ballast Tanks
The ballast tanks of bulk carriers are subjected to significantly higher levels of mechanical damage than other vessels, due to the effects of reverse impact damages. A typical example is shown in photograph 12. This type of damage is caused both by grabs, bobcats, etc operating in the holds and by external impacts from tugs and floating debris.
The reduced scantlings on the holds and the outer hull will have the effect of increasing the severity of this type of damage in double hulled design vessels. The more severe impact damage in combination with the “thermos effect” where the cargo remains at higher temperatures for the duration of the voyage, will cause considerable problems with maintenance in the ballast tanks of double hulled vessels.
The new designs of double hulled bulk carriers should enable full access, inspection and maintenance of the ballast tanks, to a greater extent than is currently available on single hulled designs. If this does not happen, there is a significant danger that rapid failures of the coating and steel may be undetected between, or even at, major surveys.
8. Maintenance of Coatings in Double Hull Areas.
The double hulled bulk carrier designs differ from their tanker counterparts in that there will be a smaller distance between the outer shell and the inner skin. This distance is expected to be in the order of 1000mm to 1400mm.
The internal stiffeners on both the shell and inner bulkhead will take up some 400mm of this small space leaving approximately 600mm of access space; just enough for a person to squeeze through. Inspection and repair of the coating in these areas will be extremely difficult and full refurbishment (using methods such as standard abrasive blasting) will be virtually impossible.
Poor accessibility, in combination with difficulties in the initial application and inspection is likely to lead to a situation where poorly applied coatings are poorly inspected and repaired, in an unusually corrosive environment.
9. Conclusions.
From the viewpoint of corrosion and coatings, any proposed legislation mandating double hulled designs for bulk carriers could be regarded as a retrograde step. Any safety or pollution advantages to these designs should be judged very carefully against the potential economic and operational problems likely to be encountered as the result of the more rapid degradation of the fabric of the hull.
Free initial telephone guidance is available on all the topics covered on this web site.
e-mail enquiries sent to the address below will be promptly answered.


